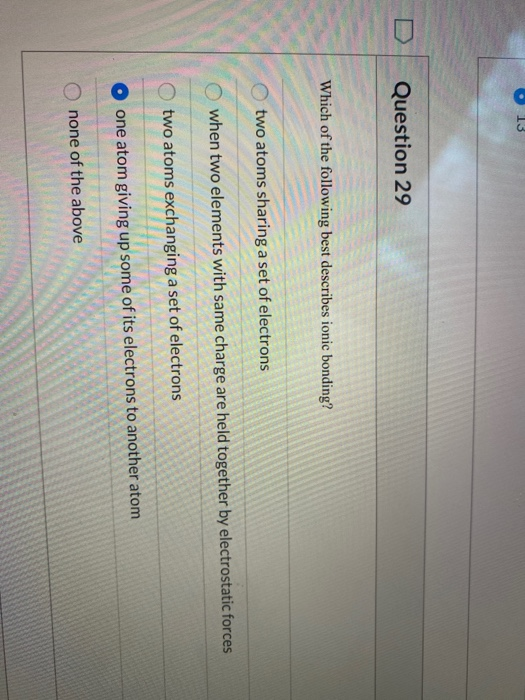
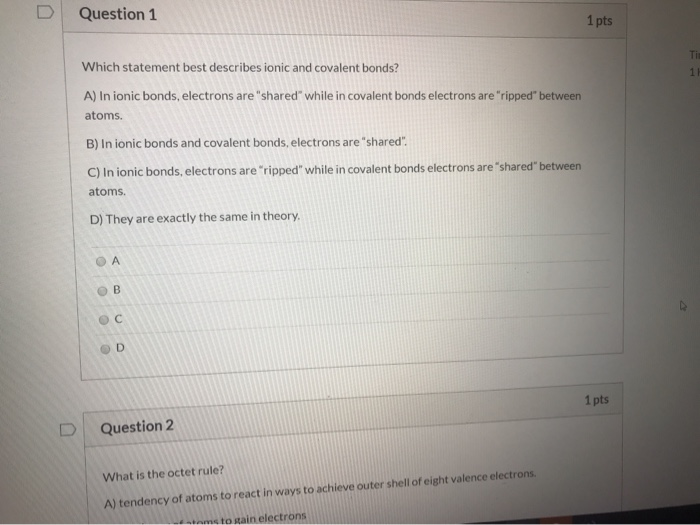
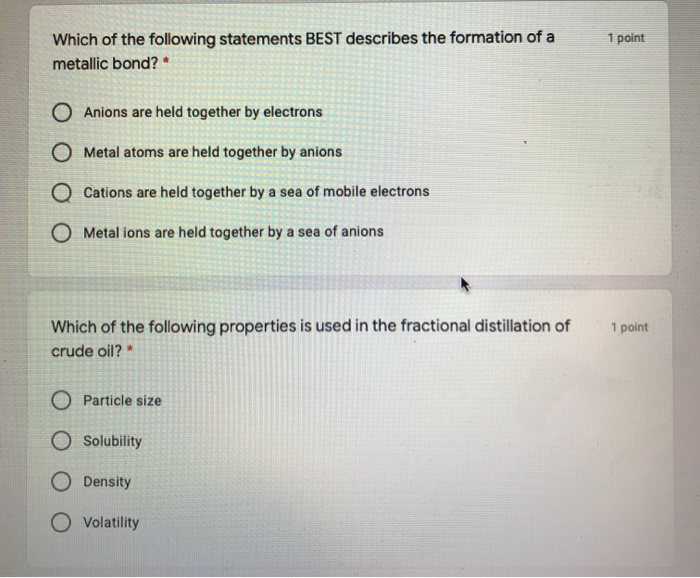
Which Of The Following Best Describes Ionic Bonding?
Which of the following best describes ionic bonding?. It has a negative charge that is spread over the entire ion. B A lithium atom shares on electron with a fluorine atom. A strong lattice of positively and negatively charged atoms held together by electrical forces B.
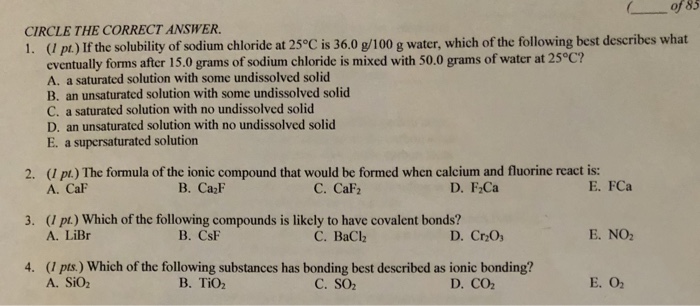
Which of the following statements best describes an ionic bond. B This is a chemical change and the molecules move farther apart. Which of the following compounds is unlikely to contain ionic bonds.
Four polar covalent bonds of which none are coordinate covalent bonds. 2 Which of the following bonds would be best categorized as covalent. Which of the following best describes the number and character of the bonds in an ammonium cation.
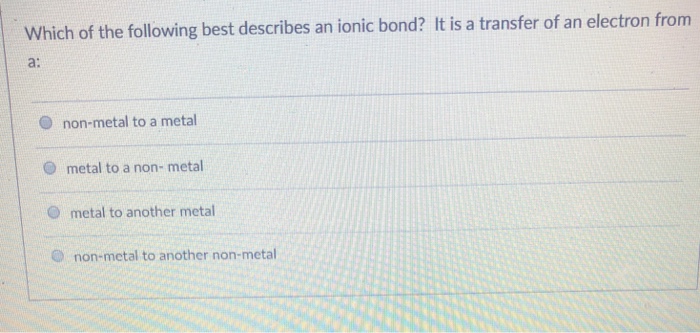
It has a nitrogen atom that is strongly attracted to Cl-. Which of the following statements best describes what happens when chocolate melts. 1An ionic bond involves a metal that exchanges electrons with a nonmetal.
Best Answer 100 1 rating An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Bacterial decomposers create glucose using the energy contained in the chemical bonds. Which of the following best describes the role of bacterial decomposition in the ocean.
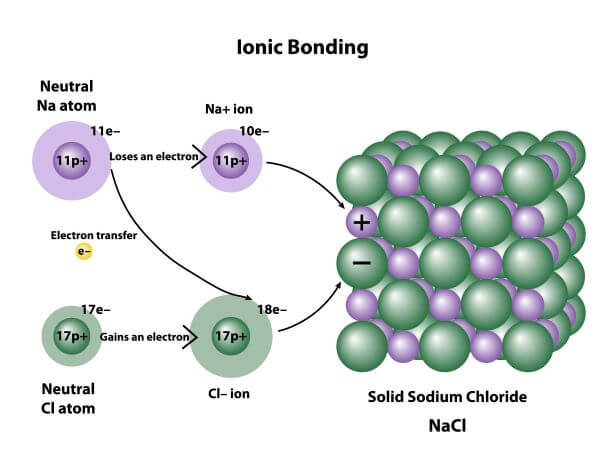
Bacterial decomposers use energy from the sun to create glucose forming the foundation of most marine ecosystems. The transfer of electrons forms strong bonds between ions. Which statement best describes ionic bonding.
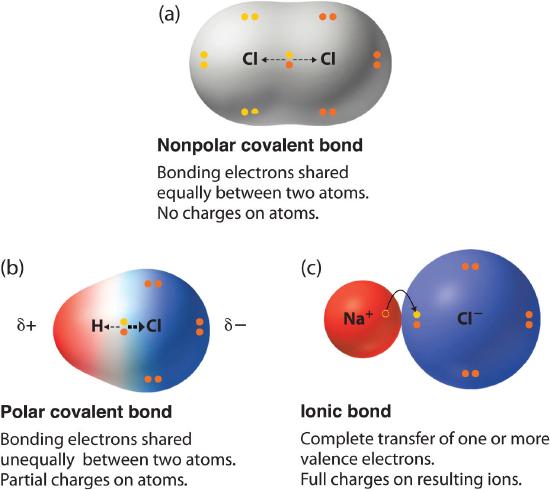
Ionic bonding is the complete transfer of valence electron s between atoms. Hydrogen bonds have ionic character.
Which of the following best describes the number and character of the bonds in an ammonium cation.
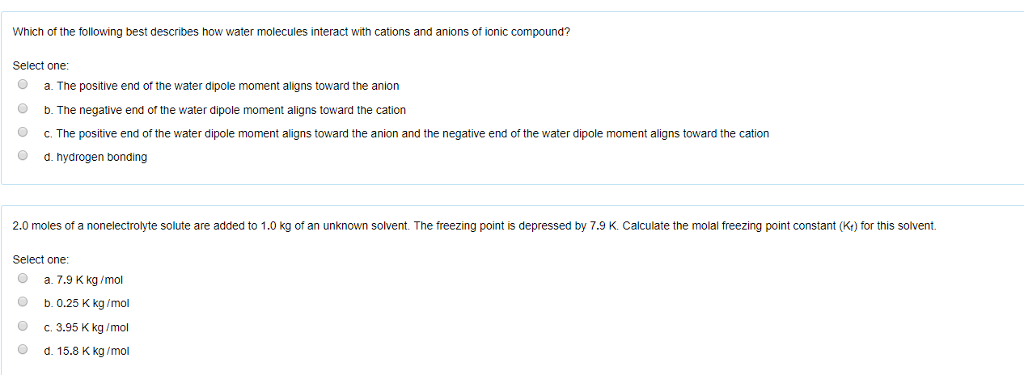
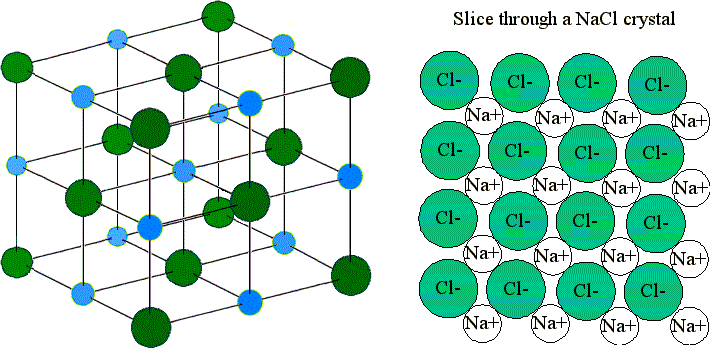
A The positive and negative charges of the ions cancel out. It has a negative charge that is spread over the entire ion. In this bond formation the atom loosing its electrons is known as electropositive atom and the atom gaining electrons is known as electronegative atom. B- there are two oxygen atoms. Hydrogen bonds have ionic character. The compounds having ionic bonds are known as ionic compounds. The bonding type present between two metal atoms C. Mc004-1jpg Which statement best describes what happens next. Which combination of shape and bond angle best describes a molecule of sulfur dioxide.
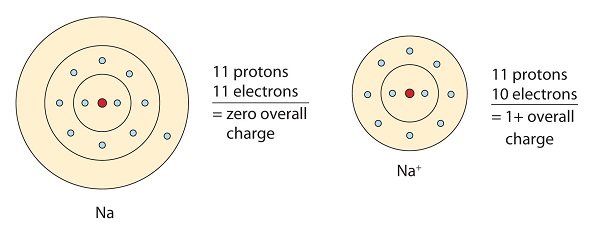
In ionic bonds the metal loses electrons to become a positively charged cation whereas the nonmetal accepts those electrons to become a negatively charged anion. In ionic bonds the metal loses electrons to become a positively charged cation whereas the nonmetal accepts those electrons to become a negatively charged anion. It has a nitrogen atom that is strongly attracted to Cl-. The transfer of electrons forms strong bonds between ions. In this bond formation the atom loosing its electrons is known as electropositive atom and the atom gaining electrons is known as electronegative atom. Van der Waals forces. The compounds having ionic bonds are known as ionic compounds.

















/1024px-Ionic_bonding.svg-589d0c0a3df78c4758805bf6.png)










Post a Comment for "Which Of The Following Best Describes Ionic Bonding?"